Biological Filtration and Aquarium Health Maintenance
Karl F. Ehrlich, Ph.D. and Marie-Claude Cantin Ph.D.
Copyright © 1995
[I have reformatted this document to be more readable and
consistent—Ed.]
Introduction
A healthy aquarium allows us to bring a fragment of the wondrous and beautiful
aquatic world into our homes or work place. Contemplation of our aquatic
microcosm brings us tranquility as greater harmony and understanding of nature's
splendid intricacies.
An aquarium may contain fishes and/or invertebrates; plants may or may not
also be present. The animals are what we invest in and whose beauty and behaviour
we appreciate; yet we confine them in an environment in which they feed and
their wastes can accumulate. The ability to maintain the animals in good
health is a function of the efficiency and health of the aquarium's wastewater
treatment facility: the biological filter and the aquarist's management of
the system.
Water purification is accomplished by teams of beneficial bacteria working
together in an appropriate environment. If the proper microorganisms are
not present or if the correct physico-chemical conditions are not maintained,
the biological filters will not function adequately. When this happens our
pets will be stressed, suffer and loose their beauty; they can even die.
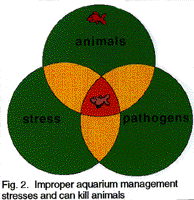 Stress is caused
by many factors including poor water quality (principally in terms of ammonia,
nitrite, pH, hardness or oxygen levels), or food (virtually always too much
and sometimes poor quality), incompatible mixtures of aquarium residents
(either intra- and/or interspecific), and/or lack of appropriate physical
environment (e.g., too bright, too much disturbance, lack of or wrong physical
substrate, no shelter) etc. Stress is cumulative; each stress combines with
another to increase the overall stress upon the individual. This applies
to not only to fishes but also to us. Just as we may quite often be exposed
to diseases without always being sick, increased stress often results in
increased susceptibility to disease; the same applies to our aquarium residents.
A properly designed and operating biological filter in a well managed aquarium
will maintain good water quality and eliminate the major stressful conditions
for aquarium animals.
Stress is caused
by many factors including poor water quality (principally in terms of ammonia,
nitrite, pH, hardness or oxygen levels), or food (virtually always too much
and sometimes poor quality), incompatible mixtures of aquarium residents
(either intra- and/or interspecific), and/or lack of appropriate physical
environment (e.g., too bright, too much disturbance, lack of or wrong physical
substrate, no shelter) etc. Stress is cumulative; each stress combines with
another to increase the overall stress upon the individual. This applies
to not only to fishes but also to us. Just as we may quite often be exposed
to diseases without always being sick, increased stress often results in
increased susceptibility to disease; the same applies to our aquarium residents.
A properly designed and operating biological filter in a well managed aquarium
will maintain good water quality and eliminate the major stressful conditions
for aquarium animals.
Beneficial microorganisms within an aquarium convert ammonia, produced by
the animals and from the breakdown of excess food and wastes, into nitrite
and then into nitrate. The cycling of an aquarium is the process of establishing
an equilibrium between the synthesis of ammonia and its conversion into nitrate
by beneficial microorganisms. City water is sterile and contains none of
the needed bacteria. Cycle Biological Supplement and Cycle Sludge Removers
have been designed to assure the presence of a balanced community of water
purifying microorganisms.
Under-gravel filters can provide a very large surface for biological filtration.
This is particularly the case when power heads are used to increase water
movement through the gravel. Once the nitrifying bacteria, in Cycle Biological
Supplement have been added, the filter will remove ammonia and nitrite.
Under-gravel filters, however, also filter the water physically, collecting
solids such as fish wastes and excess food. Efficient nitrification requires
movement of oxygenated water around the nitrifying microorganisms.
Excessive accumulation of solids, within the under-gravel filter has several
detrimental effects upon nitrification. First of all, as water movement through
the gravel, and oxygen concentration within the gravel decrease, the useful
surface area within the filter and the efficiency of nitrification or the
removal of ammonia and nitrite also decrease. Secondly, waste accumulations
in the increasingly anoxic gravel result in synthesis of ammonia, soluble
phosphorous and biochemical oxygen demand (BOD) from the sludge and production
of nitrite from partial denitrification (see
Nitrification). High BOD levels
can inhibit nitrification. The toxicity syndrome is due to the combined reduction
in nitrification coupled with increased production of ammonia and nitrite.
The transition from beneficial to detrimental conditions within an unmanaged
under-gravel filter can be quite rapid. This explains the too often observed
phenomenon of the toxicity syndrome in established aquaria, when animals
suddenly die after several months of normal operation.
Excessive accumulations of sludge, whether in an under-gravel filter or elsewhere
in an aquarium, initiate the processes leading to the toxicity syndrome.
Cycle Freshwater Sludge Removers contain communities of beneficial microorganisms
and enzymes selected for their ability to remove sludge and to prevent the
problems associated with the toxicity syndrome.
Aquarium Water Chemistry
Aquarium health management requires some understanding of water chemistry.
Information on selected important parameters is provided below.
Phosphorous - There is much talk by aquariophiles about the evils
of phosphorous. Certain realities, however, should be kept in mind.
-
Phosphorous is required by all living creatures. Phosphorous is used to build
proteins and cells. Phosphorous is used to produce energy for cell metabolism.
-
Only soluble nutrients can pass through cell membranes or plant roots.
Phosphorous must be in a soluble form, particularly soluble phosphate, to
be readily biologically available.
-
In an aerated environment phosphate tends to bind with such elements as iron,
calcium or magnesium to form insoluble salts, which are not available for
microbial or plant growth. Anoxic conditions, however, such as found in sludge
accumulations result in solubilization of these salts increasing the availability
to algae and higher plants.
-
Problems of algal proliferation occur from excess soluble phosphorous. Regular
cleaning of filters, gravel and water changes combined with use of Cycle
Sludge Removers to minimize anoxic zones will help avoid problems.
Nitrogen - There are five forms of nitrogen: gas, organic, ammonia,
nitrite and nitrate. The relationship between the different forms of nitrogen
in known as the nitrogen cycle.
Nitrogen Gas
Air is 80% nitrogen, and this gas is not toxic at normal concentrations in
water. The only problem results from supersaturation associated with changes
in operating pressure, such as when water is released from deep reservoirs
or air is sucked into a high pressure pump. Aquarium water does not usually
have this problem. Nitrogen supersaturation causes the bends in divers as
well as in fishes. Symptoms of nitrogen supersaturation include bubbles in
blood vessels and bulging eyes.
Organic Nitrogen
Organic Nitrogen is found in fish food, their wastes, sludge as well as the
animals and plants. All life consists of approximately 16% nitrogen. Proteins
can be soluble or insoluble. Protein molecules are generally too large to
pass through cell membranes and be metabolized. Certain, but not all bacteria
are able to produce enzymes, which solubilize the proteins to smaller molecules.
These can then be biodegraded by the microbes to produce cell biomass, carbon
dioxide and water. Cycle Sludge Removers not only provide this essential
part of the water purifying community but also ones which solubilize fats
and carbohydrates.
Ammonia
Fish excrete
amm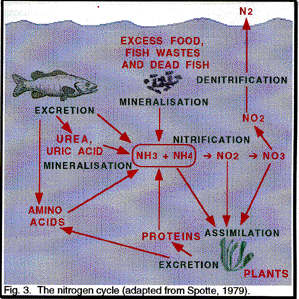 onia from
their gills. Ammonia is produced by the animals as a function of nitrogenous
wastes. This process is a part of the Nitrogen
Cycle in an aquarium. There are two forms of ammonia, unionized
(NH3) and ionized (NH4+). Only the unionized form is
toxic. An equilibrium exists in water between the quantity of NH3 and NH4+;
the percentage of the toxic form increases with the temperature and pH (Fig.
4). Thus at 25° C., in fresh water, less than 0.1% of the total ammonia
is in the toxic form, but at pH 8.5 NH3 accounts for more than 10% - an increase
of over 100 times. Ammonia is less toxic at lower pH levels. Most test kits
measure the sum of both forms of ammonia. The only way to know how much ammonia
is in the toxic form is by determining the pH and temperature and then
calculating the percentage in toxic form. The long-term safe level of ammonia
varies with different species, but the value of 0.02 ppm of NH3 is
often used. If a test kit gives a reading of 1 ppm for total ammonia at 25°
C. in fresh water this means that at pH 7.0 the toxic form is 0.6% of the
total or 1 ppm x 0.006 = 0.006 ppm NH3
onia from
their gills. Ammonia is produced by the animals as a function of nitrogenous
wastes. This process is a part of the Nitrogen
Cycle in an aquarium. There are two forms of ammonia, unionized
(NH3) and ionized (NH4+). Only the unionized form is
toxic. An equilibrium exists in water between the quantity of NH3 and NH4+;
the percentage of the toxic form increases with the temperature and pH (Fig.
4). Thus at 25° C., in fresh water, less than 0.1% of the total ammonia
is in the toxic form, but at pH 8.5 NH3 accounts for more than 10% - an increase
of over 100 times. Ammonia is less toxic at lower pH levels. Most test kits
measure the sum of both forms of ammonia. The only way to know how much ammonia
is in the toxic form is by determining the pH and temperature and then
calculating the percentage in toxic form. The long-term safe level of ammonia
varies with different species, but the value of 0.02 ppm of NH3 is
often used. If a test kit gives a reading of 1 ppm for total ammonia at 25°
C. in fresh water this means that at pH 7.0 the toxic form is 0.6% of the
total or 1 ppm x 0.006 = 0.006 ppm NH3
and there is no problem, but at pH 8.5 the toxic form is 15% of the total
or 1 ppm x 0.15 = 0.05 ppm NH3 and there is a problem.
Another factor to consider is the units that the test kit measures. Ammonia
contains nitrogen (N) and hydrogen (H). Some kits may report
to tal ammonia,
as described above, but other kits refer to the quantity of nitrogen in the
ammonia molecule. In this case the test kits units are presented as total
ammonia nitrogen (TAN), which is the sum of
NH3-N+NH4-N. Results reported in the two forms are
not the same. It is necessary to know the percentage of nitrogen in the ammonia
molecule to compare the two units. The atomic weight of nitrogen is 14 and
that of hydrogen is 1; thus the molecular weight of NH3 is 14+3
= 17. Nitrogen is 14/17 or 82% of the weight of the ammonia molecule. The
difference is much more important for nitrite and nitrate (see below). Thus
1 ppm of NH3 is the same thing as 0.82 ppm of NH3-N.
It is essential to know what units are being measured with each test kit.
The bacteria in Cycle Biological Supplement remove ammonia by converting
it to nitrite and then to nitrate (see
Nitrification below). Other strains in
Cycle Biological Supplement and Cycle Sludge Removers also consume ammonia
as a nitrogen source for their growth.
tal ammonia,
as described above, but other kits refer to the quantity of nitrogen in the
ammonia molecule. In this case the test kits units are presented as total
ammonia nitrogen (TAN), which is the sum of
NH3-N+NH4-N. Results reported in the two forms are
not the same. It is necessary to know the percentage of nitrogen in the ammonia
molecule to compare the two units. The atomic weight of nitrogen is 14 and
that of hydrogen is 1; thus the molecular weight of NH3 is 14+3
= 17. Nitrogen is 14/17 or 82% of the weight of the ammonia molecule. The
difference is much more important for nitrite and nitrate (see below). Thus
1 ppm of NH3 is the same thing as 0.82 ppm of NH3-N.
It is essential to know what units are being measured with each test kit.
The bacteria in Cycle Biological Supplement remove ammonia by converting
it to nitrite and then to nitrate (see
Nitrification below). Other strains in
Cycle Biological Supplement and Cycle Sludge Removers also consume ammonia
as a nitrogen source for their growth.
Nitrite
Nitrite is produced from ammonia as well as from nitrate. Excessive nitrite
(NO2-) is toxic to aquatic organisms particularly in fresh water.
The toxicity of nitrite is greatly reduced in saltwater. The long-term safe
level of NO2- varies with different species; 0.1 & 0.2 ppm
are considered safe limits in soft and hard water respectively. As for ammonia,
test kits give results in terms of nitrite or nitrite-nitrogen
(NO2-N). The difference between the two units is greater than
for ammonia. The atomic weight of oxygen is 16; thus the molecular weight
of NO2 is 14+32=46. Nitrogen is 14/46 or 30% of the weight of
the nitrite molecule. Thus a test kit reading of 1 ppm of NO2
is the same thing as 0.3 ppm of NO2-N. It is essential to know
what units are being measured with each test kit.
Nitrate
Nitrate is the final product of nitrification, and it tends to accumulate
in aquaria without living plants. Nitrate of even above 100 ppm have been
shown to be nontoxic to many species. Nitrate accumulation in aquaria can
be controlled by regular water changes and use of live plants. Bacteria in
Cycle are also able to convert nitrate to nitrogen gas under special conditions.

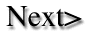
 Stress is caused
by many factors including poor water quality (principally in terms of ammonia,
nitrite, pH, hardness or oxygen levels), or food (virtually always too much
and sometimes poor quality), incompatible mixtures of aquarium residents
(either intra- and/or interspecific), and/or lack of appropriate physical
environment (e.g., too bright, too much disturbance, lack of or wrong physical
substrate, no shelter) etc. Stress is cumulative; each stress combines with
another to increase the overall stress upon the individual. This applies
to not only to fishes but also to us. Just as we may quite often be exposed
to diseases without always being sick, increased stress often results in
increased susceptibility to disease; the same applies to our aquarium residents.
A properly designed and operating biological filter in a well managed aquarium
will maintain good water quality and eliminate the major stressful conditions
for aquarium animals.
Stress is caused
by many factors including poor water quality (principally in terms of ammonia,
nitrite, pH, hardness or oxygen levels), or food (virtually always too much
and sometimes poor quality), incompatible mixtures of aquarium residents
(either intra- and/or interspecific), and/or lack of appropriate physical
environment (e.g., too bright, too much disturbance, lack of or wrong physical
substrate, no shelter) etc. Stress is cumulative; each stress combines with
another to increase the overall stress upon the individual. This applies
to not only to fishes but also to us. Just as we may quite often be exposed
to diseases without always being sick, increased stress often results in
increased susceptibility to disease; the same applies to our aquarium residents.
A properly designed and operating biological filter in a well managed aquarium
will maintain good water quality and eliminate the major stressful conditions
for aquarium animals.
 onia from
their gills. Ammonia is produced by the animals as a function of nitrogenous
wastes. This process is a part of the
onia from
their gills. Ammonia is produced by the animals as a function of nitrogenous
wastes. This process is a part of the  tal ammonia,
as described above, but other kits refer to the quantity of nitrogen in the
ammonia molecule. In this case the test kits units are presented as total
ammonia nitrogen (TAN), which is the sum of
NH3-N+NH4-N. Results reported in the two forms are
not the same. It is necessary to know the percentage of nitrogen in the ammonia
molecule to compare the two units. The atomic weight of nitrogen is 14 and
that of hydrogen is 1; thus the molecular weight of NH3 is 14+3
= 17. Nitrogen is 14/17 or 82% of the weight of the ammonia molecule. The
difference is much more important for nitrite and nitrate (see below). Thus
1 ppm of NH3 is the same thing as 0.82 ppm of NH3-N.
It is essential to know what units are being measured with each test kit.
The bacteria in Cycle Biological Supplement remove ammonia by converting
it to nitrite and then to nitrate (see
tal ammonia,
as described above, but other kits refer to the quantity of nitrogen in the
ammonia molecule. In this case the test kits units are presented as total
ammonia nitrogen (TAN), which is the sum of
NH3-N+NH4-N. Results reported in the two forms are
not the same. It is necessary to know the percentage of nitrogen in the ammonia
molecule to compare the two units. The atomic weight of nitrogen is 14 and
that of hydrogen is 1; thus the molecular weight of NH3 is 14+3
= 17. Nitrogen is 14/17 or 82% of the weight of the ammonia molecule. The
difference is much more important for nitrite and nitrate (see below). Thus
1 ppm of NH3 is the same thing as 0.82 ppm of NH3-N.
It is essential to know what units are being measured with each test kit.
The bacteria in Cycle Biological Supplement remove ammonia by converting
it to nitrite and then to nitrate (see